Prof. Lorenzo Stella is extremely proud to have been invited to become an editor for the Biophysical Journal. He will be managing articles for the Membrane section, collaborating with Associate Editor Heiko Heerklotz and many other prominent experts in the field. He strongly recommends considering the Biophysical Journal for your next publication. It is the official journal of the Biophysical Society, and a non-profit initiative at the service of the biophysical community, ensuring the highest scientific standards.

Some concentrations of antimicrobial drugs kill some bacteria, while other survive. This phenomenon can lead to the evolution of drug resistance, and the critical range of concentrations is therefore called “Mutant selection window”. The factors leading to the heterogeneous response of individual bacterial cells to antimicrobials are unknown. Genotypic or phenotypic differences could definitely play a role. However, rather surprisingly, we have observed the same phenomenon also in the case of pore formation induced in artificial vesicles by antimicrobial peptides. Liposomes are all identical in size and lipid composition, and therefore this finding was rather puzzling, and led us to wonder whether the same factors at play in the case of vesicles could contribute to the mutant selection window of bacteria, too. Our article titled “Water-membrane partition and the mutant selection window of antimicrobial peptides: insights from liposome studies” has been published in Journal of Colloid and Interface Science, and reports our findings. In brief, at least in the case of liposomes, the heterogeneous response is only apparent, and due to the fact that not all peptides are bound to vesicles. When pore formation is plotted as a function of membrane-bound peptide, vesicle leakage takes place at a well defined critical cocentration. This finding allows some suggestions for the development of new peptides with a narrower mutant selction window.

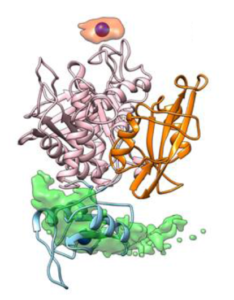
Our article titled Fluorescent Labeling Can Significantly Perturb Measured Binding Affinity and Selectivity of Peptide–Protein Interactions has been published in J. Phys. Chem. Letters., and highlighted in the issue cover. Fluorescence is widely used to quantify peptide-protein binding processes (pictured in the image). We demonstrated that peptide labeling with a fluorophore can significantly perturb the binding free-energy, in a way that depends on the specific dye and protein. Fortunately, displacement assays performed with unlabeled peptides can successfully eliminate these artifacts. Our findings are relevant for the many studies in which fluorescence is used to quantify biomolecular interactions. If you do not have access to this journal, but are interested in our article, you can download a free reprint from the following link (50 reprints are available): https://pubs.acs.org/articlesonrequest/AOR-DNPIHP2NXKRFRDDZVFGG

The master’s thesis of Dr. Chiara Innamorati, titled “Peptidomimetic inhibitors of protein-protein interactions involving the oncogenic phosphatase SHP2: spectroscopic and computational studies,” was awarded the Paliotta prize for the best Chemistry thesis in our university. The thesis was co-supervised by Lorenzo Stella and Paolo Calligari. Congratulations, Chiara!

Prof. Lorenzo Stella was listed as one of the most influential world’s scientists in an analysis performed by Stanford university.

We are happy to announce that today Prof. Lorenzo Stella was promoted to full professor. Congratulations!

Based on our patent for inhibitors of protein-protein interactions of the oncogenic phosphatase SHP2, we are working towards the foundation of a startup, named PeptoShield. Today, our business idea received several awards at the StartCup competition of the Lazio administrative region. Out of 38 proposals, we were considered the second-best researchers’ project and the best project for social impact and we also received a special award by the Lazio region. The team of PeptoShield is led by Lorenzo Stella and is highly interdisciplinary, comprising Barbara Biondi (National Research Council), Gianfranco Bocchinfuso (Tor Vergata University), Simone Martinelli (National Institute of Health) and Marco Tartaglia (Bambino Gesù childrens’ hospital), co-inventors of the patent, together with three young researchers: Chiara Innamorati (Tor Vergata University), Luca Pannone (National Institute of Health and Bambino Gesù childrens’ hospital) and Danilo Pugliese (Tor Vergata University).
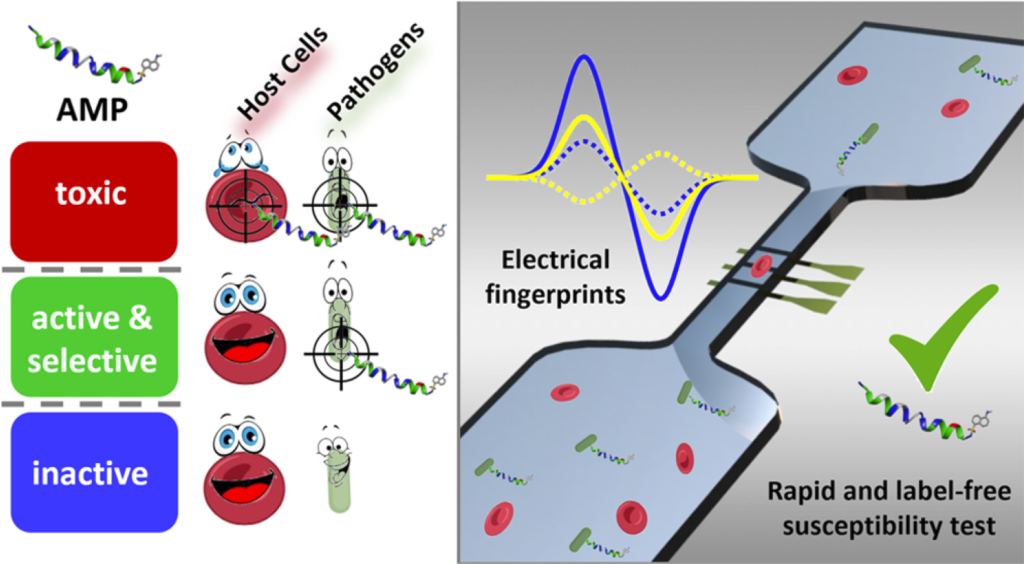
Our article titled Rapid Assessment of Susceptibility of Bacteria and Erythrocytes to Antimicrobial Peptides by Single-Cell Impedance Cytometry has been published in ACS sensors. The study was the result of a collaboration co-directed with Prof. Caselli (of the school of Engineering of our University), and involving several national and international institutions. We introduce a new, fast and label-free method to analyze the activity and cytotoxicity of antimicrobial peptides at the single-cell level, allowing experiments on mixed cell populations (bacteria and host cells present simultaneously), which better mimic the conditions encountered in vivo. We look forward to the new experiments that will be possible with this novel platform.
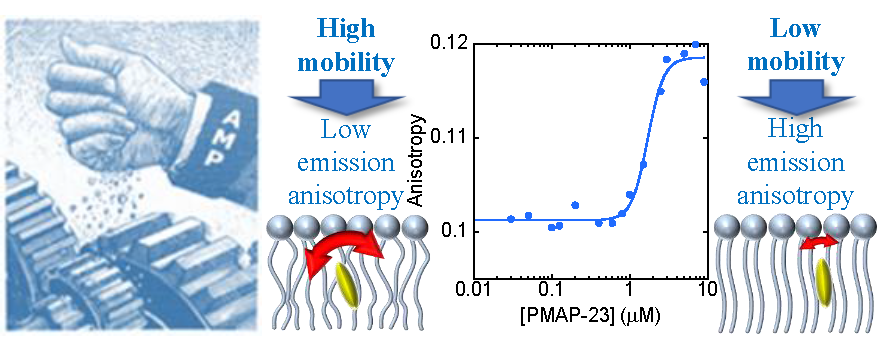
Our article titled Effects of antimicrobial peptides on membrane dynamics: a comparison of fluorescence and NMR experiments has been accepted for publication in Biophysical Chemistry (see here for free access for 50 days; permanent link is here). We demonstrate that antimicrobial peptides (AMPs) reduce the fluidity of bacterial membranes, in the nanoseconds timescale. This effect probably contributes to the bactericidal activity of these peptides, by affecting the function of membrane proteins, which is strongly dependent on the physicochemical properties of the bilayer.
Surprisingly, our data also show that fluorescence and NMR experiments provide different views of membrane dynamics, due to the very different time scales that they sample. Conclusions on membrane fluidity and order, based on a single technique should be considered with caution.
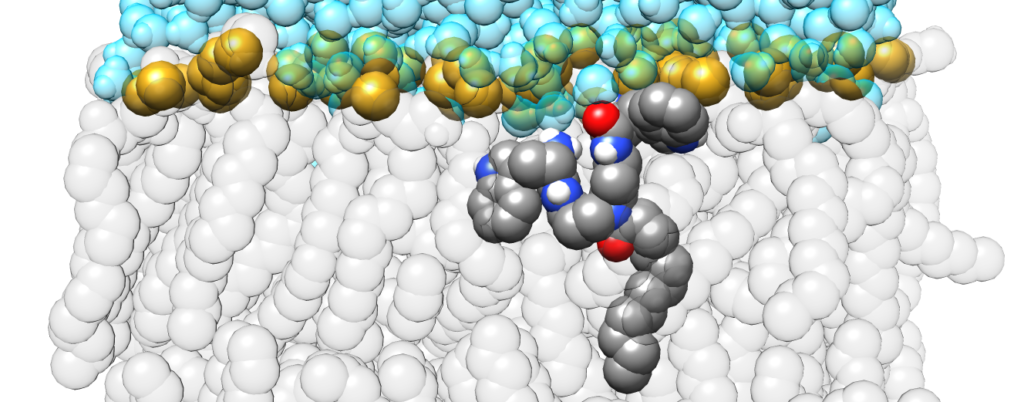
Our article titled Mechanism of lipid bilayer perturbation by bactericidal membrane-active small molecules has been accepted for publication in Biochim. Biophys. Acta Biomembranes (see the uncorrected proof here). Membrane-active small molecules (MASMs) are small organic molecules designed by the group of Prof. Jayanta Haldar, to reproduce the fundamental physicochemical properties of natural antimicrobial peptides: their cationic charge and amphiphilic character. This class of compounds has a promising broad range of antimicrobial activity and, at the same time, solves some major limitations of antimicrobial peptides, such as their high production costs and low in vivo stability. In this work, we show that, notwithstanding their simple structure, MASMs act just like antimicrobial peptides. They baccumulate on the membrane, below the head-groups, and insert their apolar moieties in the core of the bilayer, causing the formation of defects when a high coverage of the bilayer surface is reached.
 Prof. Stella was invited as a Visiting Professor at the University of Strasbourg, Institute of Chemistry, in the group of Prof. Burkhard Bechinger. His stay in France led to many possible collaborations and new friendships.
Prof. Stella was invited as a Visiting Professor at the University of Strasbourg, Institute of Chemistry, in the group of Prof. Burkhard Bechinger. His stay in France led to many possible collaborations and new friendships.

Our invention, “Peptides targeting SHP2 and uses thereof”, has been selected as one of the best technologies presented at the international event Biovaria2022. This meeting aims at connecting inventors and scientists with pharmaceutical companies and investors in the life-science sector. Our inhibitors of the protein-protein interactions of the oncogenic SHP2 phosphatase have been developed in collaboration with Gianfranco Bocchinfuso, of our Department, Ospedale Bambino Gesù (Marco Tartaglia), ISS (Simone Martinelli) and CNR (Barbara Biondi), within the framework of projects funded by AIRC and Regione Lazio.

Our Italian patent application, “Peptides targeting SHP2 and uses thereof”, has been approved. The invention describes peptide-based inhibitors of protein-protein interactions for the SHP2 phosphatase, a pivotal node in intracellular signaling. Patented applications include the therapy of cancer and of some rare diseases. An international extension has already been filed.
Our article titled “Discriminating between competing models for the allosteric regulation of oncogenic phosphatase SHP2 by characterizing its active state” has been published in the Computational and Structural Biotechnology Journal. It focuses on the SHP2 phosphatase, which is the topic of our AIRC funded project and the target of the protein-protein interaction inhibitors that we have developed and are currently patenting. By using powerful molecular dynamics computer simulations, we characterized the active state of the protein, showing that it is extremely flexible. In addition, the study provided several novel insight into the allosteric regulation mechanism of SHP2. These findings are a new step towards the development of novel anticancer drugs aimed at this pivotal molecular target.
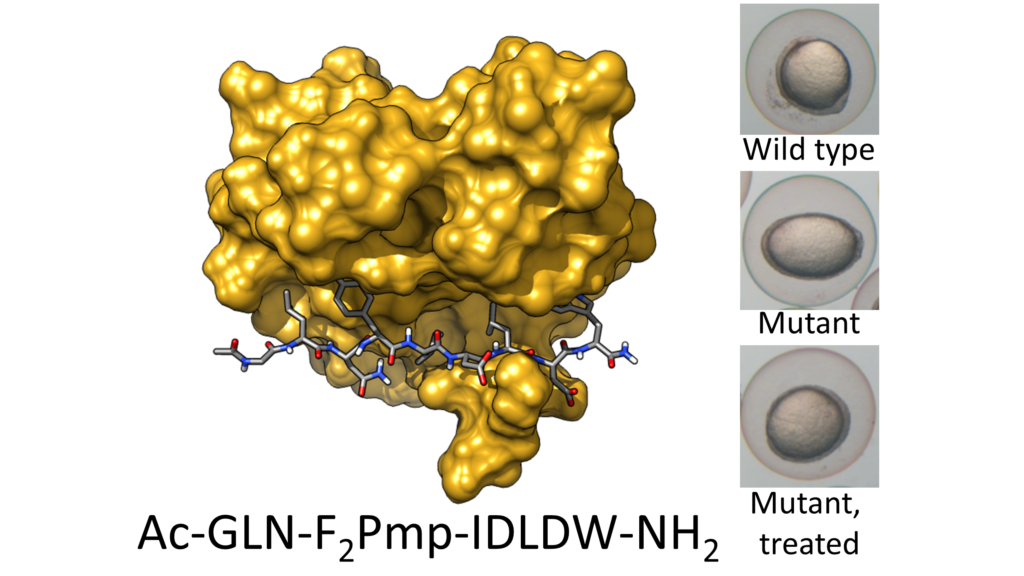
Our study, titled “Targeting Oncogenic Src Homology 2 Domain-Containing Phosphatase 2 (SHP2) by Inhibiting Its Protein–Protein Interactions” has been published in the Journal of Medicinal Chemistry. This work is the result of a wide collaboration, involving Ospedale Pediatrico Bambino Gesù, Istituto Superiore di Sanità, University of Padua, Hubrecht institute and Leiden University, in addition to our own University of Tor Vergata, and reports a new therapeutic approach for a central oncogenic target.
Protein phosphorylation is a post-translational modification involved in intracellular signaling cascades regulating fundamental processes such as cell proliferation and differentiation. Consequently, kinases (enzymes catalyzing phosphorylation) are a main target of anticancer drugs. By contrast, phosphatases (catalyzing dephosphorylation) have long be considered undruggable. Allosteric inhibitors, blocking a phosphatase called SHP2 in a closed, autoinhibited state, have recently been discovered. However, these molecules are poorly effective on SHP2 mutants that cause leukemias or genetic disorders, for which no cure currently exists. Here we demonstrate the efficacy of an alternative approach, using peptide-based molecules to inhibit SHP2 interactions with other proteins, rather than its catalytic activity. These inhibitors are particularly effective on SHP2 mutants and revert the effects of the mutations in vivo.
June 14th, 2021

Congratulations to Francesco Riccitelli, who graduated cum laude with a M.Sc. thesis titled Effects of antimicrobial peptides on the dynamics of bacterial cells. The defense was held in person, for the first time after the second wave of the Covid-19 pandemic.
May 31st, 2021

 Our project IPPO, titled “Inibitori delle interazioni proteina-proteina della fosfatasi SHP2: una strategia innovativa per la terapia oncologica e delle malattie rare“ (inhibitors of protein-protein interaction of SHP2 phosphatase: an innovative strategy for the therapy of cancer and rare diseases), funded by Regione Lazio in the framework of call “Gruppi di Ricerca 2020” and carried out together with Ospedale Pediatrico Bambino Gesù, has officially started, with the signature of a collaboration agreement between our two institutions.
Our project IPPO, titled “Inibitori delle interazioni proteina-proteina della fosfatasi SHP2: una strategia innovativa per la terapia oncologica e delle malattie rare“ (inhibitors of protein-protein interaction of SHP2 phosphatase: an innovative strategy for the therapy of cancer and rare diseases), funded by Regione Lazio in the framework of call “Gruppi di Ricerca 2020” and carried out together with Ospedale Pediatrico Bambino Gesù, has officially started, with the signature of a collaboration agreement between our two institutions.
May 21st, 2021
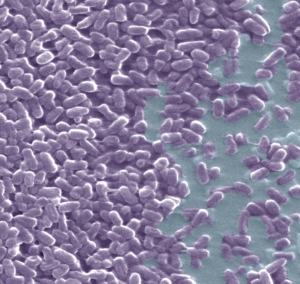
Our study, titled “Inoculum effect of antimicrobial peptides” has been published in the Proceedings of the National Academy of Sciences, USA. In collaboration with the groups of Maria Luisa Mangoni (Sapienza University of Rome) and of Henrik Franzyk (Copenaghen University) we have studied how the activity of antimicrobial peptides is affected by the density of baceterial cells.
Citing from the “Significance statement”: Bacterial drug resistance is a crucial threat to global health, and antimicrobials with novel mechanisms of action are urgently needed. Antimicrobial peptides are natural molecules that kill bacteria mostly by perturbing their membranes, and they constitute promising compounds for fighting resistant microbes. Their activity is normally tested under standardized conditions of bacterial density. However, the bacterial load in clinically relevant infections varies by many orders of magnitude. Here, we show that the minimum peptide concentration needed for bacterial growth inhibition can vary by more than 100-fold with an increase in the density of cells in the initial inoculum of the assay (a phenomenon termed the “inoculum effect”). These findings question the utility of the currently used activity screening assays.
A preprint of the first version of the manuscript can be accessed here (although the paper improved significantly during the review process).
April 21st, 2021

Our research on peptide-based inhibitors of protein-protein interactions of the SHP2 phosphatase was highlighted on National television, with an interview to Lorenzo Stella and Gianfranco Bocchinfuso. You can find the whole video here. We would like to take this occasion to thank AIRC, PRACE, CINECA and Regione Lazio for supporting our group and all the people who have collaborated to this research, in particular the co-inventors of our patent application, Marco Tartaglia (OPBG), Simone Martinelli (ISS), Barbara Biondi (University of Padua). These results would not have been possible without the efforts of all our postdocs and students who have worked on the project (Sara Bobone, Paolo Calligari, Massimiliano Anselmi, Vivana Canale, Annalisa Bortolotti, Valerio Santucci, Andrea Quercioli, Giuseppe Torini, Cristiano Di Stefano). Just to set the record straight, the Nature paper mentioned by the journalist is not by our group.
April 15th, 2021

Administrative region Lazio founded our project IPPO to develop our patent application on peptide inhibitors of the SHP2 phosphatase for the treatment of rare diseases and hematologic malignancies.
January 1st, 2021

Today we start a new project! The Italian Foundation for Cancer Research (AIRC) has funded for 5 years our project titled “Inhibitors of SHP2 protein-protein interactions: a new strategy for a crucial oncogenic target“. Thank you AIRC!!
November 15th, 2020

Very sad news. Our mentor Basilio Pispisa passed away. A great scientist and a great man. A large part of our research, to this day, was originally inspired by his suggestions. We will miss him dearly.
September 24th, 2020

Congratulations to Giulia Perilli and Francesca Mazzotta, who graduated in the first defense session held in person, after the lockdown.
May 19th, 2020
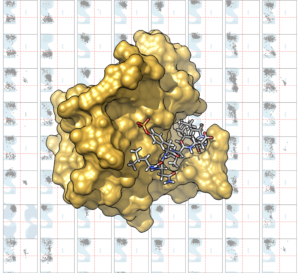
Our paper titled “Structural Determinants of Phosphopeptide Binding to the N-Terminal Src Homology 2 Domain of the SHP2 Phosphatase” has been published by the ACS Journal of Chemical Information and Modeling. By performing several μs long molecular dynamics simulations, we identified the structural determinants of high affinity/specificity ligands to the N-SH2 domain of SHP2. SH2 domains are protein-protein interaction modules involved in many signaling pathways, and therefore represent potential pharmacological targets in cancer therapy.
May, 13th, 2020
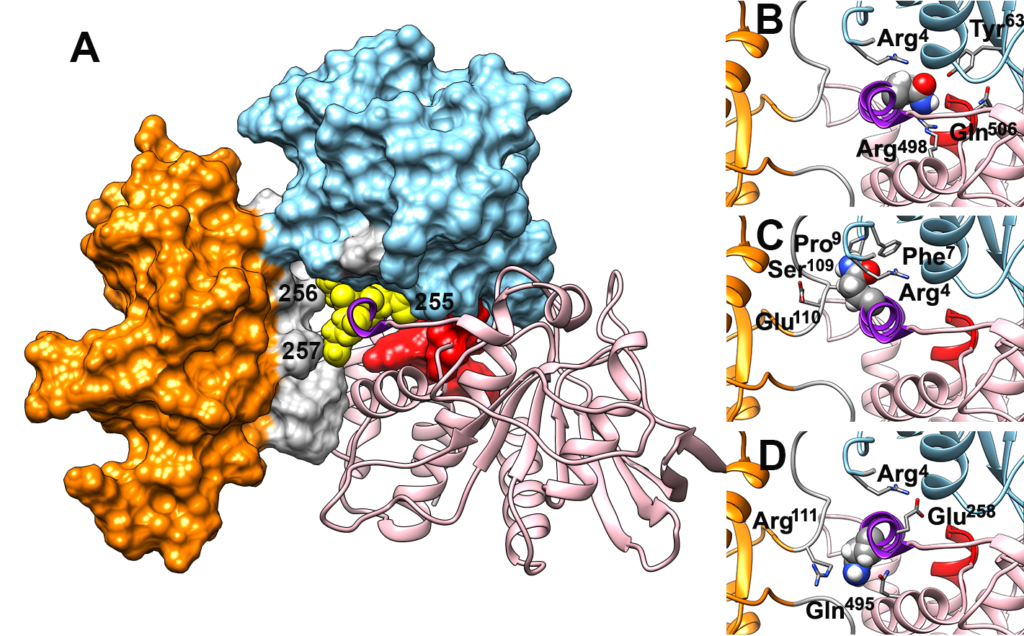
In collaboration with several institutions (Istituto Superiore di Sanità, Ospedale Pediatrico Bambino Gesù, University, Magdeburg, University of Nicosia) and with Prof. Gianfranco Bocchinfuso, of our Department, we elucidated the molecular effects of pathogenic mutations causing a rare genetic disease (Noonan syndrome). Our findings are published in the journal Human Mutation, in an article titled Pathogenic PTPN11 variants involving the poly‐glutamine Gln255‐Gln256‐Gln257 stretch highlight the relevance of helix B in SHP2’s functional regulation.
April, 28th, 2020

Antimicrobial peptides (AMPs) are bactericidal and immunomodulatory molecules that kill bacteria mostly by perturbing their membranes, and for this reason they are a promising class of molecules to fight drug-resistant microbes. Our current understanding of AMPs has been
reached by two parallel, but largely independent, approaches: microbiological studies on AMP
effects on cells, and biophysical investigations on model membranes. In the last few years,
several groups, including ours, are trying to bridge the two worlds, characterizing quantitatively peptide behavior with biophysical methods directly in real cells. Together with Miguel Castanho, Sattar Taheri-Araghi and Sergey A. Akimov, Prof. Lorenzo Stella is editing a special issue focused on this interdisciplinary area that is changing the field of AMPs. Several authors have already caccepted to participate, but submissions are open!
April 14th, 2020

Congratulations to Paolo Calligari and Sara Bobone, members of our group, for their controbution to the fight against the COVID epidemics. Their article can be downloaded here.
March 28th, 2020

Bacteria act as a heroic soldier who covers an exploding grenade with his body to save his comrades in arms.
Our article titled Binding of an antimicrobial peptide to bacterial cells: Interaction with different species, strains and cellular components has now been accepted for publication in Biochimica Biophysica Acta: Biomembranes and is online. In collaboration with the groups of Marialuisa Mangoni (Sapienza University of Rome) and Yoonkyung Park (Chosun University) we have extended our previous studies quantifying the interaction of antimicrobial peptides (natural molecules able to kill drug-resistant superbugs) with bacterial cells. We found that millions of peptide molecules must accumulate on each cell to kill it. Part of them interact with the cell membranes, while a fraction binds to other cellular components. After a bacterium is dead, it binds twice as many peptides, thus protecting the other cells from the antimicrobial action of these molecules.
An open access post-print can be downloaded here
November 1st, 2019
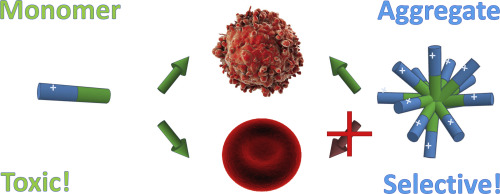
Our article titled Aggregation determines the selectivity of membrane-active anticancer and antimicrobial peptides: The case of killerFLIP has been accepted for pubblication in Biochimica Biophysica Acta: Biomembranes. We have shown that aggregation can reduce the driving force for binding to the externally neutral membranes of healthy eukaryotic cells, by reducing the water exposure of hydrophobic moieties present in amphiphathic peptides. At the same time, self-assembly does not affect significantly peptide affinity for the negatively charged bilayers of cancer cells and bacteria, which is mostly driven by electrostatic interactions. Our data indicate that aggregation should be taken into account, or even exploited, in the design of selective anticancer and antibacterial membrane-active peptides.
July 4th, 2019

Prof. Basilio Pispisa, founder and mentor of the physical chemistry group at the University of Tor Vergata, was awarded the Bonino medal, the highest award of the Physical Chemistry division of the Italian Chemical Society. Congratulations, Prof. Pispisa!
May 20th, 2019

The book “Antrimicrobial peptides: basics for clinical applications“, edited by Prof. Katsumi Matsuzaki, is fresh off the press. We contribuited with a chapter titled “Selectivity of Antimicrobial Peptides: A Complex Interplay of Multiple Equilibria”
An open access post-print can be downloaded here.
March 20th, 2019

The fab four: today, our Master’s students Elisa Grelloni, Andrea Quercioli, Giuseppe Torini and Simone Bonacorsi defended their theses and graduated in Chemistry, all of them cum laude!
Elisa’s thesis regards a method to increase the activity and selectivity of antimicrobial peptides; Andrea and Giuseppe worked on the characterization of the oncogenic phosphatase SHP2 and Simone discussed his studies on novel antimicrobial peptidomimetics. Congratulations!
March 8th, 2019
 I am afraid that something is going terribly wrong with the scientific publishing industry, peer reviewing and the ethics of the scientific community as a whole. In the last years, I was surprised to find a huge number of published papers containing extremely basic and fundamental errors that completely undermine the article’s conclusions and that should have been spotted by a decent reviewing process. Together with my friend and colleague Marco van de Weert, I wrote several articles* trying to improve this situation, at least in our own research field. It is not difficult to imagine our frustration when we find, more and more often, that our articles are cited in support of the very same mistakes that we tried to correct. We discuss this issue in a paper titled “The danger of citing papers you did not read or understand” that has just been accepted for publication in the Journal of Molecular Structure.
I am afraid that something is going terribly wrong with the scientific publishing industry, peer reviewing and the ethics of the scientific community as a whole. In the last years, I was surprised to find a huge number of published papers containing extremely basic and fundamental errors that completely undermine the article’s conclusions and that should have been spotted by a decent reviewing process. Together with my friend and colleague Marco van de Weert, I wrote several articles* trying to improve this situation, at least in our own research field. It is not difficult to imagine our frustration when we find, more and more often, that our articles are cited in support of the very same mistakes that we tried to correct. We discuss this issue in a paper titled “The danger of citing papers you did not read or understand” that has just been accepted for publication in the Journal of Molecular Structure.
*References:
March 5th, 2019
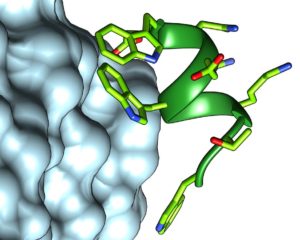 Our article titled “Rational Design of Antiangiogenic Helical Oligopeptides Targeting the Vascular Endothelial Growth Factor Receptors” has just been accepted for publication in Frontiers in Chemistry. In the framework of a national collaboration involving the universities of Milan, Padua, Insubria and the National Research Council, we have developed short helical peptides, inspired by the N-terminal helics of VEGFC, that show antiangiogenic activity in the nM range and are resistant to proteases. These molecules are promising candidates for the development of novel antiangiogenic drugs with applications in anticancer therapy and in ophthalmology. The article is part of a Research Topic titled “Folded Synthetic Peptides for Biomedical Applications“.
Our article titled “Rational Design of Antiangiogenic Helical Oligopeptides Targeting the Vascular Endothelial Growth Factor Receptors” has just been accepted for publication in Frontiers in Chemistry. In the framework of a national collaboration involving the universities of Milan, Padua, Insubria and the National Research Council, we have developed short helical peptides, inspired by the N-terminal helics of VEGFC, that show antiangiogenic activity in the nM range and are resistant to proteases. These molecules are promising candidates for the development of novel antiangiogenic drugs with applications in anticancer therapy and in ophthalmology. The article is part of a Research Topic titled “Folded Synthetic Peptides for Biomedical Applications“.
February 22nd, 2019
 Annalisa Bortolotti defended her PhD thesis, titled From peptides to peptidomimetics: spectroscopic studies of membrane-active antimicrobial agents. Congratulations, Dr. Bortolotti!
Annalisa Bortolotti defended her PhD thesis, titled From peptides to peptidomimetics: spectroscopic studies of membrane-active antimicrobial agents. Congratulations, Dr. Bortolotti!
December 4th, 2018

Our review titled “From liposomes to cells: Filling the gap between physicochemical and microbiological studies of the activity and selectivity of host‐defense peptides” was selected for inclusion in a joint virtual issue of ChemBioChem, ChemMedChem and Peptide Science. These three journals picked for the special collection their top articles on peptides published in the past year. All these articles are free to read until March 2019.
October 9th, 2018
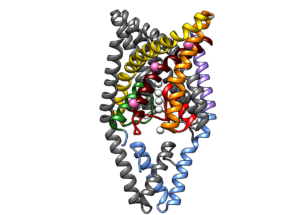 In the framework of an international collaboration coordinated by Ospedale pediatrico Bambino Gesù, our group contributed to the discovery of a new rare disease (that we named FHEIG, an acronym summarizing the main clinical features of the syndrome). Our study, published in the American Journal of Human Genetics revealed that FHEIG syndrome is caused by mutations in the gene coding for the TRAAK potassium channel. Our molecular dynamics simulations showed that mutations perturb the conformational transitions regulating the channel’s responsivenes to external stimuli. Understanding the molecular basis of a rare disease is important for early diagnosis and is the first step in the search for new diagnostic approaches.
In the framework of an international collaboration coordinated by Ospedale pediatrico Bambino Gesù, our group contributed to the discovery of a new rare disease (that we named FHEIG, an acronym summarizing the main clinical features of the syndrome). Our study, published in the American Journal of Human Genetics revealed that FHEIG syndrome is caused by mutations in the gene coding for the TRAAK potassium channel. Our molecular dynamics simulations showed that mutations perturb the conformational transitions regulating the channel’s responsivenes to external stimuli. Understanding the molecular basis of a rare disease is important for early diagnosis and is the first step in the search for new diagnostic approaches.